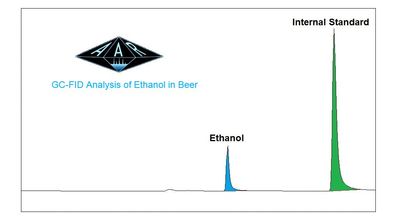
 There are many methods used for the determination of ABV (Alcohol by Volume), which one suits your needs? Are you starting a quality program? Not happy with your current laboratory? Wanting to achieve accuracy and precision with your label claim or nutritional facts panel vs ongoing production? "Why not align your quality program with the testing methods used by regulatory agencies?" Makes sense right? The TTB (Alcohol and Tobacco Tax and Trad Bureau) has set out to explain their testing protocol to verify label claims in their 2004-1 ruling. Within the ruling they define what procedures will be used to verify calories, alcohol, carbohydrates, and protein. These are mostly AOAC and several in-haus TTB procedures. For ABV verification of Standard Malt Beverages, Flavored Malt Beverages, Standard Wines, Ciders, and Sake, and Flavored Wines they utilize the AOAC technique of Gas Chromatography-Flame Ionization Detection or (GC-FID). At AAR we have chosen to align our testing protocols with those of the 2004-1 ruling including the GC-FID procedure for ABV testing. These procedures are also aligned with FDA Menu testing for nutritional facts, an added bonus. In an ever evolving market of products containing non-traditional ingredients and utilizing new brewing techniques, we feel this gives everyone involved the best chance for success. There are many advantages to the GC-FID technique, many of them revolve around the disadvantages of others. Let's look at chromatography or (separation) versus spectroscopy. The most widely used technique of spectroscopy (NIR or RI) takes a look at the absorbance spectrum of the entire sample (ethanol + matrix). Matrix for our purposes here is everything else in the sample that's not ethanol. So, in spectroscopy, a one point standard of water/ethanol is used to calibrate the absorbance of the instrument. How does it account for the matrix effects, or separate them, or eliminate their impact on the results? In chromatography, we can use a multi-dimensional approach to eliminating interferences. Meaning we are are looking at "apples to apples." The sample is introduced into the "inlet" where our first dimension goes to work. The temperature is selective to the boiling point of ethanol, so we can already leave behind many compounds that are not volatile, or have higher boiling points, such as, carbohydrates, sugars, organic acids, etc. even before they enter our second dimension of separation the "column." The compounds are further separated based on their chemical qualities and interaction with the stationary phase (column) and pushed through to the detector by the mobile phase (gas). In addition, the use of external standards for every run is key for keeping all samples/standards on a level playing field, thus allowing many other analytical issues to fall away. The result of all this is we get to look directly at the ethanol "peak" in a standard versus an ethanol "peak" in the sample. Apples to apples... In the case of sample vs standard, an internal standard is also used to further level the playing field, with 5 standard concentrations used, you know that the sample is bracketed, linearity is evaluated and all samples are run in duplicate. Any poor duplications (>5% diff) are re-tested. On a 4.2% ABV labeled product, our analysis resulted in a %RSD of 1.52 (N=16). Therefore our analysis produced values from 4.14% to 4.26% ABV, all well within the 0.3% acceptance range by TTB. Even more importantly, using the same method as regulatory agencies to evaluate your product gives a greater chance of success. AAR has been using this technique and we have great success on a wide variety of matrices, non-alcoholic, low-alcohol, wine, ciders, and more. Through separation we can achieve precision and accuracy, eliminating the matrix effects found using other techniques. Cheers, ZL
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorZach Lilla, Lead Chemist at AAR has over 17 years of analytical chemistry experiece focused in the areas of Food, Dietary Supplements, Beer, Wine, Hops, and Distillate Analysis. Archives
August 2022
Categories |
Don't Like the Shopping Cart?
|

 RSS Feed
RSS Feed